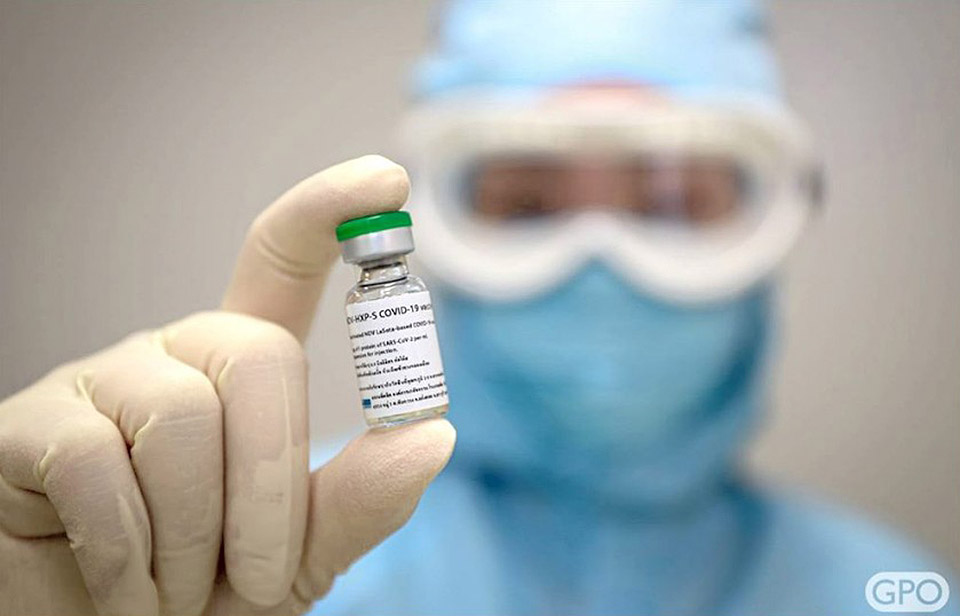
The Government Pharmaceutical Organisation (GPO) kicked off the first phase of human trials for its COVID-19 vaccine on Monday by delivering shots to 18 volunteers.
The trials are being conducted in cooperation with Mahidol University’s Faculty of Tropical Medicine.
The GPO has been developing its COVID-19 vaccine by cultivating the inactivated virus in hen eggs, before extracting the culture to make the vaccine.
The GPO said the first phase of human trials will cover 210 volunteers aged between 18 and 59, who will be given the smallest dose of the vaccine.
The second phase, which will cover 250 volunteers aged between 18 and 75, will get a slightly higher dose.
Researchers hope to determine the correct dosage based on these studies.
The second phase is expected to kick off in July and the GPO expects to conclude the study by the end of the year.
All volunteers in the first and second phases are expected to have good health, no drug or vaccine allergies and should never have contracted COVID-19.
This vaccine trial is supported by the US-based Program for Appropriate Technology in Health (PATH), which has provided vaccine precursors for first and second-phase studies.





