Washington (AP) – A powerful new technology holds the promise of rapidly altering genes to make malaria-proof mosquitoes, eliminate their Zika-carrying cousins or wipe out an invasive species, but a report Wednesday says these “gene drives” aren’t ready to let loose in the wild just yet.
Advisers to the government say lots more research is needed to learn to safely use gene drives and understand the ecological and social consequences of essentially hijacking evolution, spreading genetic changes through populations of insects, animals or certain plants faster than nature.
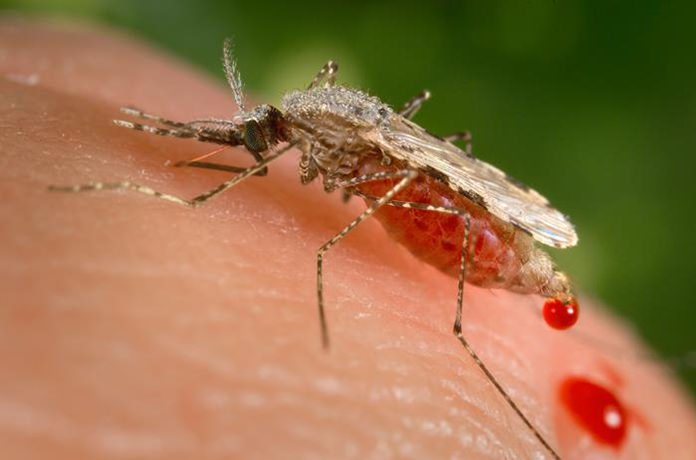
And the public, around the world, needs a say in whether and how gene drives eventually are used, especially because some may be intended for low-income countries, stressed the National Academies of Sciences, Engineer and Medicine.
“Public engagement cannot be an afterthought,” said Vanderbilt University medical ethicist Elizabeth Heitman, who co-chaired the National Academies’ committee.
Gene drives are on the horizon. Already, a California lab has hatched mosquitoes that spread a malaria-blocking gene every time they reproduce. Researchers say it should be possible to eliminate populations of another mosquito – the kind that spreads the Zika virus and dengue fever – by making them sterile.
Similarly, gene drives one day might be used to wipe out invasive species such as invasive rodents that devastate native plants and animals on many of the world’s islands, without toxic chemicals. Or they might help reverse pesticide resistance in a crop-suffocating weed.
“The gene drive approach could offer a safer, less expensive and more lasting solution” to many problems, said National Academies’ committee member Jason Delborne, an associate professor of science, policy and society at North Carolina State University.
But the report called for cautious, phased-in research given questions about the technology’s impact. It said gene drives should be studied first in laboratories before moving to tightly controlled field trials – in greenhouses, screened cages or even on remote islands, to lower any risk of escape – to help determine if organisms modified in this unique way ever should be released into the environment.
The National Institutes of Health, which requested the report, welcomed the findings.
“This approach to potential irreversible modification of the genome of an entire species is breathtaking,” said NIH Director Dr. Francis Collins. But the call for cautious research “seems to strike the right balance, given both the exciting potential of this technology and uncertainty about its ecological impact,” he added.
Don’t confuse gene drives with the many other genetic technologies that scientists have long used, the panel cautioned. For example, a biotech firm wants to test in the Florida Keys mosquitoes altered to produce offspring that can’t survive outside of a lab. That alteration used traditional genetic engineering, meaning any population decline would be slower and more controllable than if gene drives had been used.
What’s the difference?
Normally, genes have a 50-50 chance of being inherited. Gene drives bias that inheritance, allowing scientists to genetically modify an organism to carry a particular trait and then ensure it spreads to virtually all its offspring. It only works in certain kinds of fast-reproducing species, but it means entire populations could be affected in only a few generations.
Scientists have known this occasionally happens in nature as some species inherit certain genes at higher-than-expected rates. For half a century, they’ve tried to harness that biological power. But recently, that research has surged thanks to a gene-editing technique named CRISPR that allows precise editing of DNA in living cells.
No one knows how rapidly altering entire populations could affect habitats. What if wiping out invasive species lets something worse fill that empty niche? What happens if an alteration spreads to an unintended species?
Moreover, gene drives would spread with no regard for national borders, the panel warned. It called for international scientific and regulatory collaboration, and noted that even in the United States, it’s not clear who’s in charge.
Consider those island rodents: Would a gene drive in a mouse be governed as an animal drug by the Food and Drug Administration, or a rodent poison by the Environmental Protection Agency, or a plant pest by the Agriculture Department?
Some leading researchers already are tackling these concerns. At the University of California-Irvine, molecular biologist Anthony James hopes to begin field trials of his malaria-proof mosquitoes in a few years and is working on the science, regulatory and ethical steps needed so that “people agree we’ve done it (the) right way.”
Massachusetts Institute of Technology gene-drive pioneer Kevin Esvelt praised the recommendations, but also urges researchers to publicly disclose all their experiments.
“Gene drive systems are intrinsically about altering the shared environment. We should at the very least have the courtesy to inform people what is being planned, and let them voice their opinions, before we begin,” he said.




