Washington (AP) – California researchers hatched some malaria-resistant mosquitoes and then gave evolution a shove – using a groundbreaking technology to ensure the insects pass on that protective gene as they reproduce, with implications far beyond the promise of fighting malaria.
The experiment reported Monday involves what’s called a “gene drive,” a technique that, if it pans out, promises to alter the genetics of populations of insects and certain plants and animals faster than Mother Nature could.
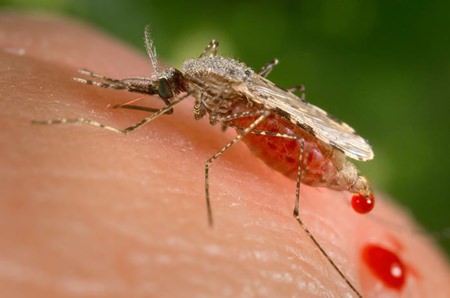 This photo provided by the Centers for Disease Control and Prevention (CDC) shows a feeding female Anopheles stephensi mosquito crouching forward and downward on her forelegs on a human skin surface, in the process of obtaining its blood meal through its sharp, needle-like labrum, which it had inserted into its human host. California researchers hatch malaria-resistant mosquitoes and use a groundbreaking technology to ensure the insects pass on the protective gene as they reproduce. It has implications far beyond fighting malaria. (James Gathany/CDC via AP)
This photo provided by the Centers for Disease Control and Prevention (CDC) shows a feeding female Anopheles stephensi mosquito crouching forward and downward on her forelegs on a human skin surface, in the process of obtaining its blood meal through its sharp, needle-like labrum, which it had inserted into its human host. California researchers hatch malaria-resistant mosquitoes and use a groundbreaking technology to ensure the insects pass on the protective gene as they reproduce. It has implications far beyond fighting malaria. (James Gathany/CDC via AP)
Normally, genes have a 50-50 chance of being inherited. University of California researchers created a strain of mosquitoes that could pass a specially engineered malaria-blocking gene to about 99 percent of their offspring.
The mutant mosquitoes, kept in a secured lab, highlight the promise of this technology along with questions about when and how it might be safe to try it in the wild.
“This is a major advance because it shows that gene drive interventions will likely be effective in mosquito vectors of disease,” said biologist Kevin Esvelt of Harvard’s Wyss Institute for Biologically Inspired Engineering, a gene drive researcher who wasn’t involved with the newest study.
But because no one knows how such rapid genetic change might impact habitats, Esvelt has urged the public to weigh in. The California study published online in Proceedings of the National Academy of Sciences adds some urgency.
“This work suggests that we’re a hop, skip and jump away from actual gene drive candidates for eventual release” in nature, he said.
Malaria kills more than half a million people a year, mostly children in Africa, and sickens about 200 million more. Mosquitoes pick up the parasite by biting an infected person, and spread it when they bite someone else. Mosquito-killing insecticides and bed nets are the main protection.
At the University of California-Irvine, molecular biologist Anthony James is developing what he calls “sustainable technologies” – rather than killing mosquitoes, instead rendering them unable to infect people.
James engineered immune system genes that could spur a mosquito’s body to develop antibodies to attack the parasite, so that it couldn’t transmit the infection. The new genes worked as intended when injected into the eggs of a particular malaria-spreading mosquito species, Anopheles stephensi.
Altered mosquitoes would have to gradually spread their new genes by mating with wild mosquito populations – and the next challenge is how to speed that process quickly enough to make a dent in malaria in any given region.
Enter gene drives, a technique that proponents say one day might be used to wipe out invasive species like kudzu or cane toads, or reverse pesticide resistance in weeds, or suppress insect populations. The idea comes from a few examples in nature where certain genes spread disproportionately, and scientists have longed for a way to control that process. Recently they’ve had some success using a powerful new tool named CRISPR-Cas9 that allows precise editing of DNA in living cells, sort of like cut-and-paste software.
Earlier this year, University of California, San Diego, biologists Ethan Bier and Valentino Gantz announced a CRISPR-fueled gene drive that worked in fruit flies.
For Monday’s study, the San Diego researchers teamed with James – packing the malaria-resistance genes with the CRISPR-based gene drive, boosting chances of inheriting the malaria protection by targeting the change to a specific spot in the mosquito’s reproductive DNA. To measure, they tacked on a fluorescence gene that made mosquitoes’ eyes look red if they harbored the new gene.
The malaria protection spread remarkably well, proving the concept even though far more work is needed before this kind of mosquito could be tested in the wild, James said. Among the findings to be addressed was that the transgenic male mosquitoes passed their new trait to the next generation more efficiently than transgenic females did.
Gene drive experiments are controversial. One worry is the possibility of altered organisms escaping the laboratory before scientists know how to use them. The California team took safeguards including special lab security and using a mosquito species that can’t survive in California’s climate.
Additional questions involve what’s appropriate to try – wiping out a species or just altering it, for example – and how to approach such research in low-income countries.
The prestigious National Academy of Sciences is studying ethical issues surrounding gene drive research, and the California team says countries that struggle with mosquito-borne diseases in particular should be involved. “Somebody sitting in the U.S. making up a list of rules has to appreciate that these countries have their own concerns,” James said.




