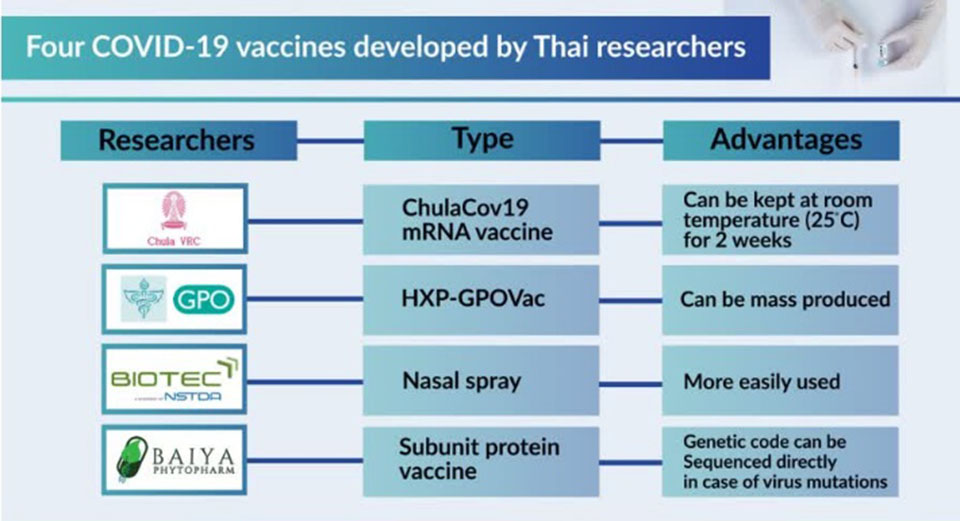
Thailand is developing multiple types of vaccine and most of them are entering into human trials this year. Today we’ll look into 4 types showing the most promising results.
The first type is made-in-Thailand mRNA vaccine developed by the Vaccine Center of Chulalongkorn University. It was previously tested in volunteers in the first phase and shows 94% efficacy, as good as other global mRNA types. The result shows that it can successfully suppress 4 strains including Alpha, Beta, Gamma and Delta and induces T-cell responses as well.
The advantage of such a type is that it can be refrigerated (2-8C) for up to 3 months and stored at room temperature (25C) for 2 weeks, making it easier to store than other mRNA vaccine brands. It also has only slight side effects.
The second human trial is expected this month, with manufacturing expected in October 2022
The second type is inactivated HXP-GPOVac developed by the Government Pharmaceutical Organisation with its international partners. It uses inactivated and genetically modified Newcastle disease virus (NDV) to express HexaPro or a stabilized spike protein of the coronavirus on the surface, to enable the human immune system to recognize and to defend itself against the infection.
This new vaccine has the advantage of being capable of cultivation in embryonated eggs, a method also used in flu vaccine manufacture; thereby making the manufacturing process inexpensive. The GPO says the vaccine can be produced at the GPO’s industrial-scale plant, resulting in around 20-30 million doses per year.
With a second human trial being conducted, the GPO expects to begin manufacturing the vaccine in the middle of 2022.
The third type is a nasal spray type developed by The National Center for Genetic Engineering and Biotechnology (BIOTEC). It can be sprayed into the nasal cavity through a special syringe designed to deliver the vaccine directly to the mucous membrane of the upper respiratory system, where most of the viruses including COVID-19 form, before being distributed all over the body. This vaccine has two kinds – Adenovirus-based, and Influenza-based.
With its inhaling function, it can be used more easily than the injected kind.
The first clinical human trial is expected to be conducted at the end of this year and manufacturing may start in the middle of 2022.
The last type is a vaccine developed by pharmaceutical startup called Baiya Phytopharm, in collaboration with the Faculty of Pharmacy of Chulalongkorn University. The vaccine uses tobacco leaves as plant platform to express antigens and be made into the vaccine.
As this is a vaccine using a plant platform, in case of future mutations of the virus, researchers can take the genetic code of new strains to produce a vaccine immediately and there’s no need to wait for an imported one.
It is now planning the first human trial and If successful, the third quarter of the year 2022, will be the first month of production. (NNT)
 |
 |
 |





