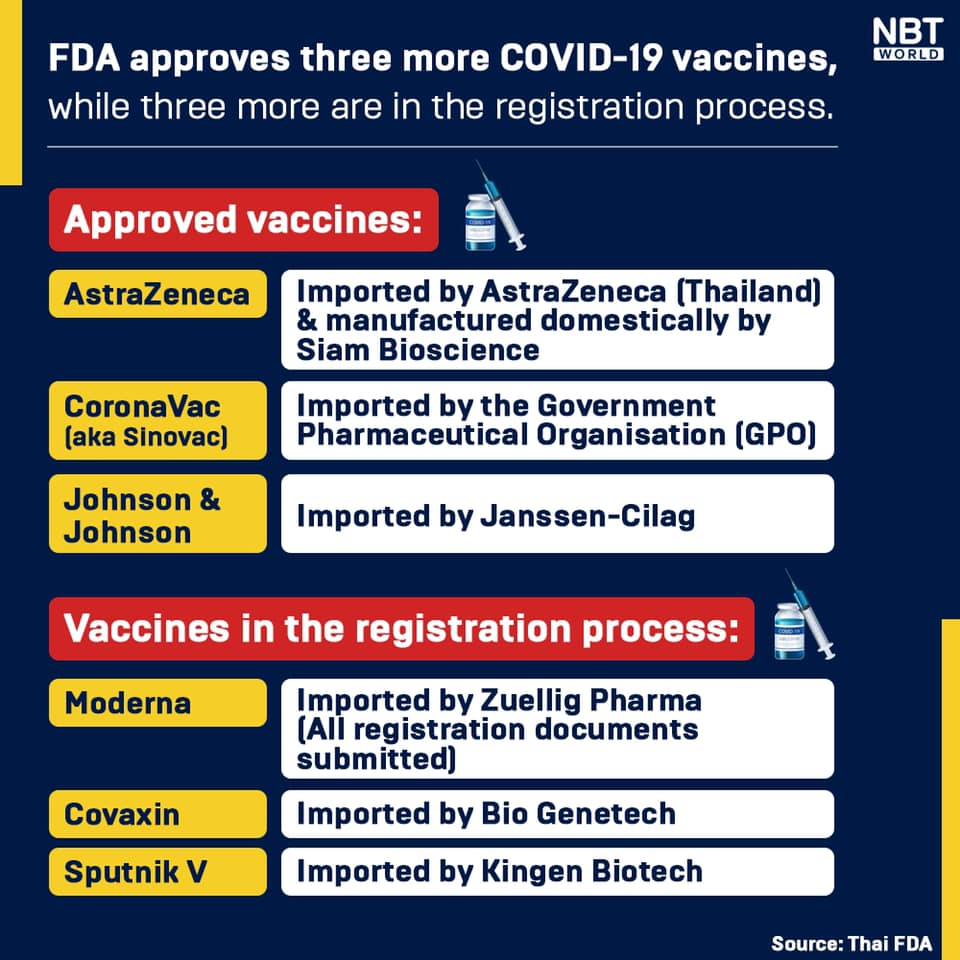
FDA secretary-general Dr Phaisan Dankhum said the Food and Drug Administration (FDA) has so far approved three Covid-19 vaccines for use in Thailand, while three more companies are in the process of registration.
Dr Phaisan said Moderna has submitted all required documents last week, and its COVID-19 vaccine should be registered this month.
The FDA has approved three COVID-19 vaccines, including AstraZeneca, Sinovac’s CoronaVac and Johnson & Johnson. The administration is now receiving documents for Covaxin vaccine, by Biogenetech, and Sputnik V vaccine by KinGen Biotech.





