
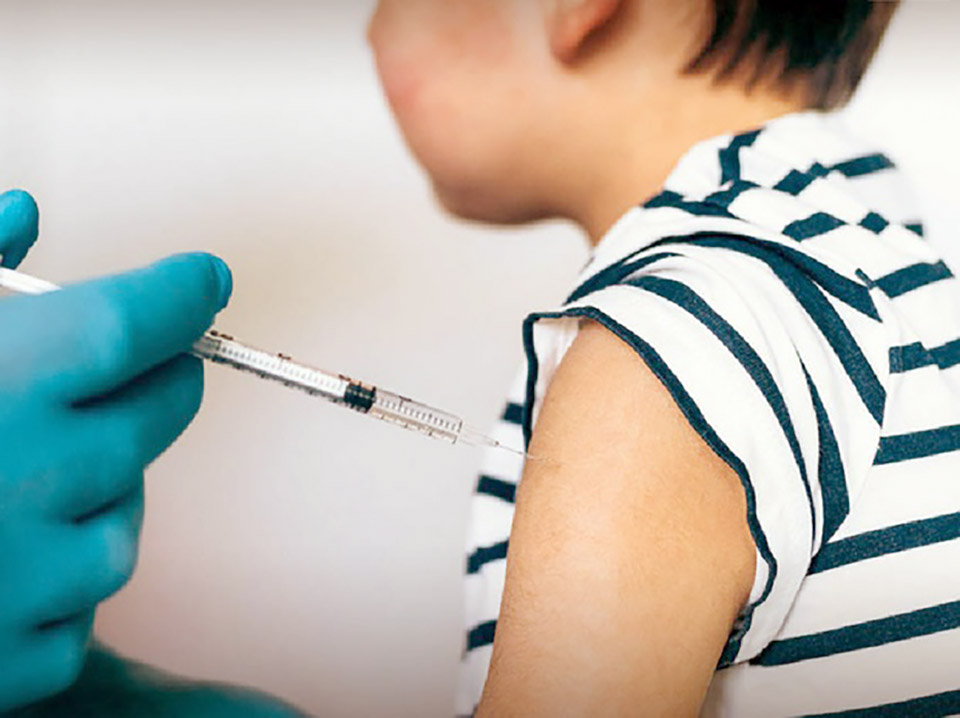
The government has outlined strategies for immunizing children against COVID-19, including acquiring Pfizer for children and awaiting approval for the use of the Sinovac vaccine for young children next month.
Dr Taweesilp Visanuyothin, Spokesperson for the Center for COVID-19 Situation Administration (CCSA), said the government is working to revise its contract with Pfizer to procure 2.9 million COVID vaccine doses for children aged 6 months to 4 years. The contract adjustment would be a swap for 3.5 million doses that Pfizer had yet to deliver for recipients aged 12 and up as initially contracted, with the remaining 590,000 doses to be for children aged 5-11.
Dr Taweesilp said the Food and Drug Administration (FDA) has yet to approve the Pfizer vaccine for young children, but said the 3.5 million doses under the contract could still be administered to children aged 5-11.
The CCSA spokesperson also said the Government Pharmaceutical Organization is currently requesting FDA approval for the use of the Sinovac vaccine for children with parents who prefer inactivated virus vaccines. The FDA is expected to make its decision by next month. (NNT)
 |
 |
 |





