
The National Center for Genetic Engineering and Biotechnology (BIOTEC) developed inhaled COVID-19 vaccines of adenovirus-based and influenza-based types to prevent infections, reduce side effects and combat mutations.
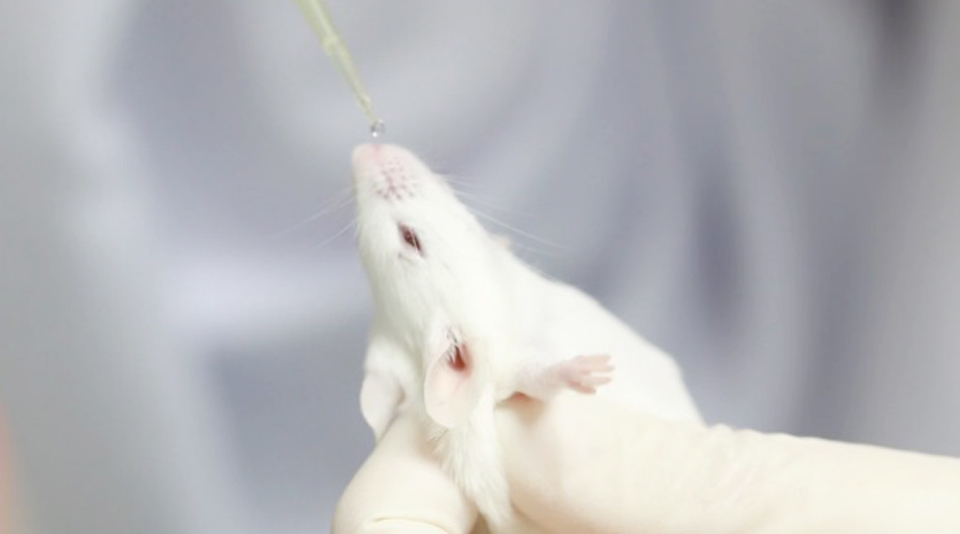
Dr Anan Jongkaewwattana, director of BIOTECH’s Veterinary Health Innovation and Management Research Group, said the vaccines passed their trials with animals and proved to be effective without a side effect.
BIOTECH will seek permission from the Food and Drug Administration to proceed with human trials together with the Chulabhorn Royal Academy to test their efficacy with the Delta variant of COVID-19.
If FDA gives quick approval, the first phase of human trials can begin late this year and the second phase can follow in March next year. If the human trials are successful, the vaccine production for inoculation can start mid-2022, Dr Anan said.
According to him, the inhaled vaccines will be administered through special inhalers that will send vaccine droplets to stimulate antibodies right in mucous membrane at the upper respiratory tract. They also reduce the risks of blood clots. BIOTECH is capable of developing a new vaccine to cope with a new variant of the virus in only a few weeks. (TNA)

 |
 |
 |





